Empower Your Gut for Better Health: A Navigation Guide
The "gut" refers to the gastrointestinal tract, which includes the stomach, intestines, and colon. Gut health, also called gastrointestinal (GI) health, is a fundamental pillar of women’s overall well-being, influencing far more than just digestion. It plays a central role in key areas such as energy production, mood regulation, immune function, and metabolic health. The GI tract is a complex network that contains not only structural components, but also integrates microbial, immune, and neuroendocrine systems. When any part of this gut network is disrupted, the consequences can extend throughout the body, often manifesting as fatigue, mood disturbances, increased susceptibility to infections, and chronic illness.
Optimal gut health relies on the dynamic interplay between the gut’s structural integrity, immune responses, neuroendocrine signaling, and the gut microbiome—a diverse community of trillions of microorganisms that live in the digestive tract. These microbes, along with their genetic material, form an intricate ecosystem essential for digestion, nutrient absorption, immune modulation, and overall health and well being. When imbalances occur in this microbial ecosystem, they can lead to systemic issues such as low energy, emotional instability, and heightened illness risk. Emerging research supports a holistic approach to gut health that includes targeted dietary strategies, lifestyle modifications, microbiome-supportive therapies, and mental health support.
For women in midlife, gut health becomes particularly vulnerable due to hormonal changes during perimenopause and postmenopause. Declining estrogen levels—can disrupt gut motility, increase intestinal permeability (commonly referred to as “leaky gut”), and lead to detrimental shifts in the composition of the gut microbiome called dysbiosis, a state of microbial imbalance. These changes may worsen digestive symptoms like bloating, constipation, and diarrhea, and lead to total body inflammation and disruption of the immune system. Therefore gut dysbiosis, marked by a reduction in beneficial, short-chain fatty acid-producing bacteria and an increase in pro-inflammatory microbes, can compound health risks across multiple systems.
The drop in estrogen at midlife also affects the estrobolome, a specialized group of gut microbes involved in metabolizing estrogen. When the estrobolome is disrupted, circulating estrogen levels can decline further, contributing to a range of health concerns including metabolic imbalances, increased cardiovascular and metabolic risk, bone loss, and systemic inflammation.
Hormone replacement therapy (HRT) adds another layer of complexity, as it can also influence GI function in ways that are not yet fully understood. Despite the significance of these issues, high-quality research specifically examining the relationship between menopause and gut health remains limited, underscoring the need for further scientific investigation.
Digestive complaints are prominent in perimenopausal women and often co-occur with mood and cognitive symptoms. The gastrointestinal symptoms that most commonly worsen during perimenopause due to fluctuating estrogen levels are abdominal bloating, altered bowel habits (including constipation and diarrhea), and symptoms consistent with irritable bowel syndrome (IBS). Increased flatulence and heartburn are also frequently seen. While symptoms should always be addressed, additional evaluation is warranted if gut issues are associated with features such as weight loss, gastrointestinal bleeding, or anemia. In these cases prompt assessment to exclude malignancies is important. Changes in gut motility and alterations in the gut microbiota associated with menopause may contribute to these symptoms. Psychological factors, including stress, tension, and anxiety, are significant contributors to the severity of both constipation and diarrhea during the menopause transition, often more so than reproductive hormone levels themselves.
Gut disorders are more prevalent in women than men, with symptom patterns and severity often influenced by menopausal status and hormonal changes. Perimenopausal women experience more fluctuating and severe GI symptoms, particularly bloating and bowel habit changes, while postmenopausal women have more persistent constipation and pelvic floor symptoms, with less symptom variability. These differences are closely linked to the hormonal milieu, especially the transition from fluctuating to consistently low estrogen levels
Racial and ethnic differences exist in the prevalence and severity of GI symptoms among midlife women, but menopausal status remains a strong independent predictor of symptom burden across groups. Overall, midlife women are at increased risk for a spectrum of GI symptoms, with the most common being constipation, bloating, abdominal discomfort, heartburn, and IBS-like complaints
Your Gut an Owner’s Guide
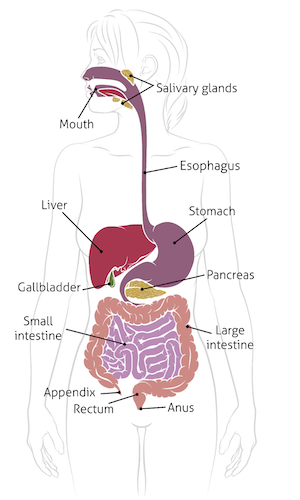
The adult gastrointestinal (GI) tract includes the esophagus, stomach, small intestine, and colon—which all coordinate to mechanically and chemically digest food, absorb nutrients, and expel digestive byproducts. The gut has increasingly recognized vastly broader roles in health and wellness. These multiple roles range from immune response to mental health. The gut’s structure and function are supported by several interconnected systems to impact our health, including the immune system and the nervous system.
Motility
The gut is in constant forward motion to mix and propel its contents for nutrient absorption and metabolism. This forward motion is regulated by the autonomic nervous system, a part of the peripheral nervous system controlled by the brain that is responsible for regulating involuntary bodily functions not only such as heart rate, blood pressure, respiration, glandular secretions, but also digestion. The autonomic nervous system coordinates gut motility and involves a near constant ongoing forward movement known as peristalsis. Interruptions in this forward propulsion, also called gut motility, can contribute to conditions like irritable bowel syndrome (IBS), which is especially common among women and features symptoms such as abdominal pain, bloating, and irregular bowel patterns.
The Intestinal Barrier
The intestinal barrier (also known as the gut barrier or intestinal mucosal barrier) refers to the complex, multi-layered structure in the intestines that acts as a selective barrier. It allows the absorption of essential nutrients, water, and electrolytes from the intestinal lumen (the inside of the gut) into the bloodstream, while preventing the entry of harmful substances such as pathogens, toxins, and undigested food particles This barrier is crucial for maintaining overall health, supporting immune function, and protecting against infections and inflammation. Disruption of barrier function ("leaky gut") is implicated in the development of a range of illnesses, including inflammatory bowel disease (IBD), metabolic syndrome, and immune-mediated disorders.
The Microbiome
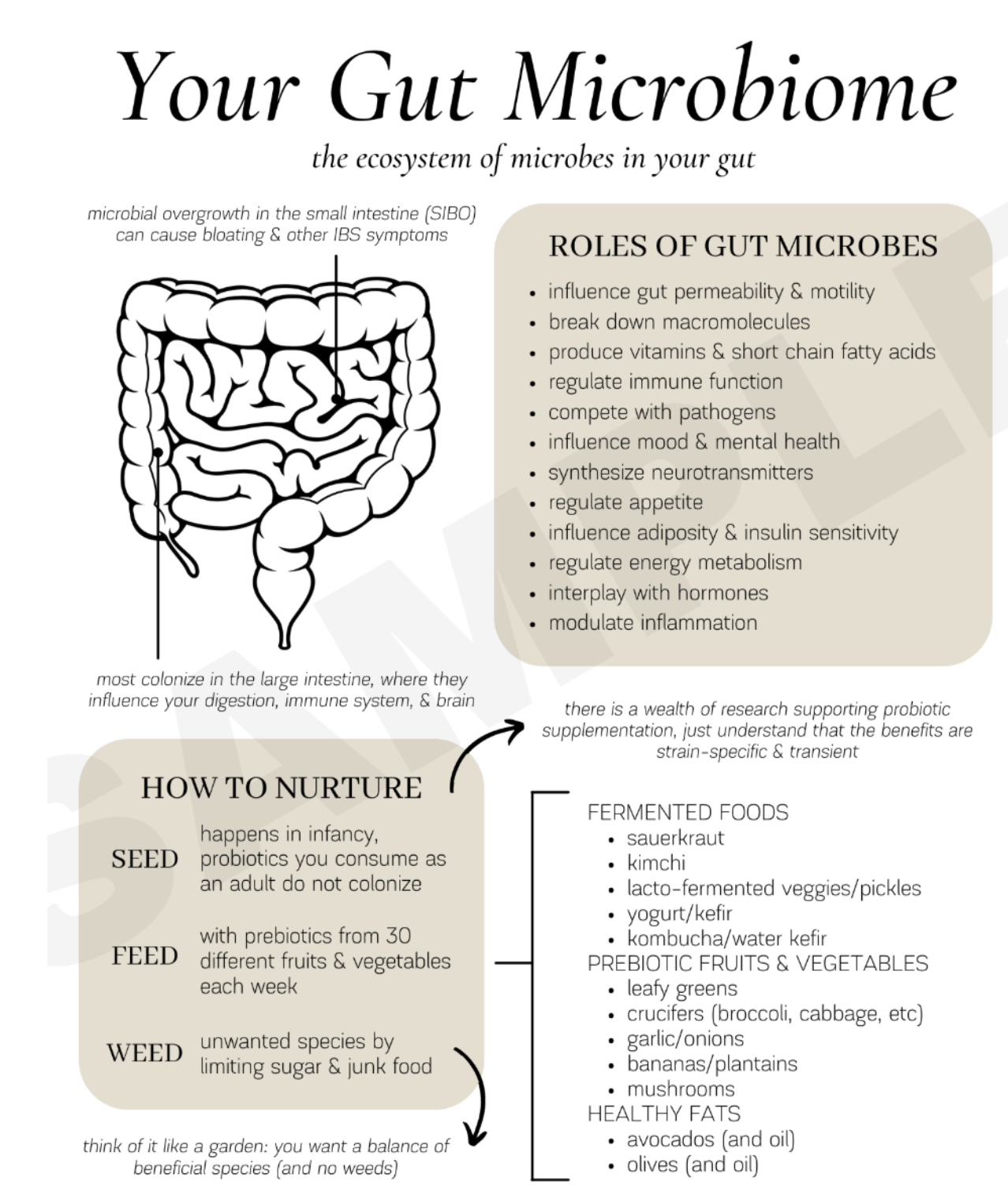
The adult GI tract harbors is also the home to a dense and diverse community of microorganisms called the microbiome, that helps to regulate overall health and wellness ranging from cognitive function and metabolism to cancer prevention. The microbiome is composed of trillions of microorganisms the live inside the gut. While bacteria dominate the microbiome, it also includes other types of microbes, making it a diverse ecosystem that can vary significantly between individuals based on factors like diet, age, geography, and lifestyle. These microorganisms of the microbiome include bacteria, viruses, fungi, and protozoa.These microbes produce metabolites like short-chain fatty acids from fermenting indigestible fibers, which support gut barrier integrity and reduce inflammation and contribute to barrier function. Dysbiosis, or disruption of the normal microbiota, is associated not only with GI disorders, but also extraintestinal diseases such as obesity and metabolic syndrome, amongst other issues. Diets rich in fiber, plant-based foods, and diverse nutrients support a healthy microbiota, enhance barrier function, and reduce inflammation. Western-type diets high in fat, sugar, and processed foods are associated with dysbiosis and leaky gut.
The gut microbiome is integral to great gut health and overall health and wellness. The composition and metabolic activity of the gut microbiota influence not only local GI function but also systemic physiological processes. Dysbiosis is associated with metabolic dysfunction, chronic inflammation, and mood disorders. The gut microbiota synthesize and modulate the availability of neurotransmitters such as serotonin, GABA, dopamine, and glutamate, which are critical for mood regulation and cognitive function. Dysbiosis is associated with increased risk of mood disorders, including depression and anxiety and dietary patterns, particularly those high in fiber and low in processed foods, promote a healthy microbiota and are associated with improved energy, mood, and immune responses.
The Immune System
The gut-associated lymphoid tissue (GALT) is the largest mass of immune tissue in the body and plays a pivotal role in defending against pathogens while maintaining harmony with beneficial microbes and dietary components. These cells are located in the walls of the gut and mobilized when needed. The majority of the body's immune cells—approximately 70-80%—are housed in the gut, making it the largest immune organ. This concentration enables rapid responses to potential threats from ingested food, water, and environmental exposures, helping to clear pathogens and prevent infections from taking hold.
As with everything in the human body, it's all connected. The gut immune system is intricately linked with the trillions of bacteria and other microbes residing in the microbiome of the gut. These microbes train and regulate immune cells, promoting a balanced response that fights harmful invaders without overreacting to harmless substances. For instance, a healthy microbiome supports immune tolerance, reducing the risk of allergies, autoimmune diseases, and chronic inflammation by educating immune components to distinguish between self, food antigens, and good bacteria. Disruptions here can lead to "leaky gut" and systemic issues, underscoring its role in overall homeostasis.
Diet directly influences this system through its effects on the microbiome; for example, certain fatty acids feed gut bacteria, producing byproducts that enhance immune cell development and function. This interplay extends to broader health outcomes, including improved resistance to infections and better cancer immunotherapy responses, and reduced inflammation-related diseases.
In essence, a well-functioning gut microbiome and immune system is foundational to robust immunity, metabolic health, and disease prevention, highlighting why factors like nutrition, probiotics, and lifestyle choices that support gut health are increasingly emphasized in medical research.
The Gut Brain Axis
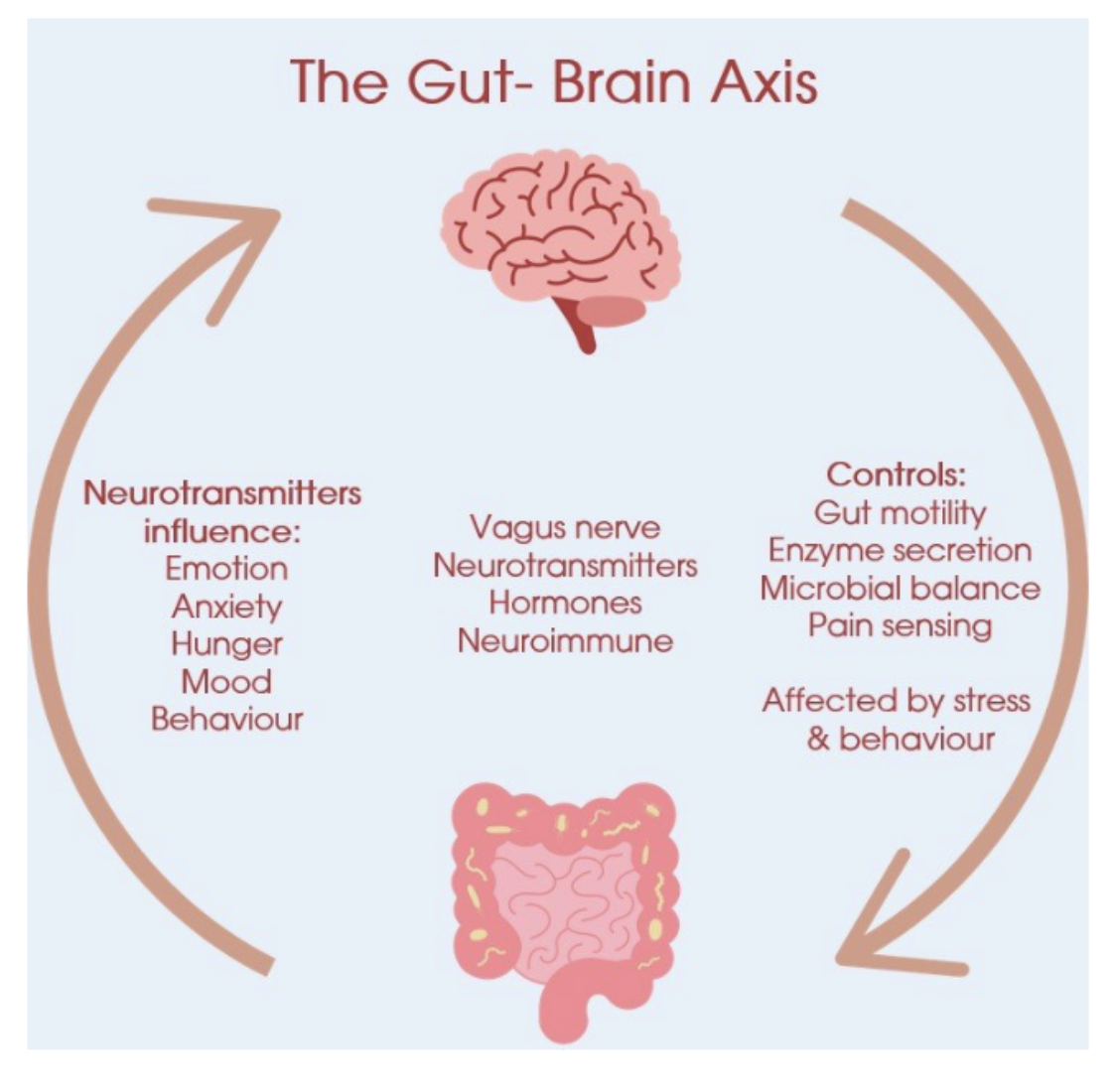
Beyond the microbiome and immune functions, the gut is closely linked to the brain through the gut-brain axis (GBA). This axis is a bidirectional communication network linking the GI tract and the central nervous system via neural, endocrine, and immune pathways. This axis regulates GI motility, secretion, mood, stress responses, and cognitive function. The GBA is a key mediator of the relationship between GI health and psychological well-being. For example, gastrointestinal disorders like irritable bowel syndrome (IBS) can trigger brain-related symptoms such as fatigue or pain perception, while stress can worsen gut motility. Emerging evidence also ties the function of the gut to neurodegenerative diseases like Parkinson's and Alzheimer's, where gut microbiota alterations may impact the brain.
The GBA plays a crucial role in overall health for several reasons. The GBA influences emotions, stress responses, and cognitive function. Gut microbes produce neurotransmitters like serotonin, about 95% of which is made in the gut, that affect brain signaling, potentially contributing to conditions such as anxiety and depression Disruptions in this axis have been linked to higher risks of mood disorders, with research showing that an imbalanced gut microbiome can exacerbate mood symptoms. Gut-derived serotonin, activates vagal signals and influences CNS serotonergic pathways, affecting mood and stress responses. The vagus nerve transmits sensory information from the gut to the brain, modulating emotional and cognitive processes.
Large-scale epidemiological studies demonstrate robust associations between GI symptoms and mood disorders. For example, adults with GI symptoms such as diarrhea, constipation, and abdominal discomfort have significantly higher odds of moderate-to-severe depressive symptoms. Interventions targeting the gut microbiota, such as high-prebiotic diets, have been shown to improve mood, anxiety, stress, and sleep in adults with moderate psychological distress. Probiotic supplementation, particularly with Lactobacillus and Bifidobacterium strains, may also benefit mood and stress, though results are strain-dependent. Large cross-sectional studies show that adults with GI symptoms have significantly higher odds of depressive symptoms. Interventional studies demonstrate that dietary interventions, such as high-prebiotic diets, can improve mood, anxiety, stress, and sleep. Probiotic supplementation may also benefit mood, though effects are strain-specific.
In addition to serotonin pathways, the GBA integrates intestinal function with brain activity via the microbiome's impact on the immune system. Gut bacteria generate metabolites that support immune responses and reduce inflammation, which in turn protect against illnesses. It connects gut issues to neurological conditions and vice versa.
Figure 1: The GI Tract
Figure 2: The Gut Microbiome
Figure 3: The Gut Brain Axis
GI Health and Energy Regulation
GI health is intimately linked to energy levels through not only nutrient absorption, but also through the microbiome and gut brain axis. The organisms of the microbiome ferment dietary fibers to produce energy substrates such as SCFAs (short chain fatty acids) which contribute to systemic energy pools, and dysbiosis can lead to metabolic diseases such as obesity. Endocrine cells located in the gut, called enteroendocrine cells, sense luminal nutrients and secrete hormones that regulate appetite, satiety, gastric emptying, and energy expenditure by acting on the brain and peripheral tissues. The rate of GI transit, or how long food stays in the gut, also influences nutrient absorption and glucose responses to ingestion of food. Slower transit times, often modulated by dietary fiber intake, can improve glucose homeostasis and reduce the risk of metabolic diseases.
Dietary patterns such as the Mediterranean diet, rich in fiber, plant-based foods, and healthy fats, are associated with increased microbial diversity, enhanced SCFA production, and anti-inflammatory effects, supporting both GI and systemic health.
The Female Gut at Midlife
The female gut at midlife undergoes notable shifts in its microbiome, the community of trillions of bacteria, viruses, fungi, and other microbes residing in the intestines. These changes are largely driven by declining levels of ovarian hormones like estrogen and progesterone, which influence microbial diversity, composition, and function. While the gut microbiome remains essential for digestion, immune regulation, hormone metabolism, and overall health, midlife alterations can contribute to dysbiosis (imbalance) and heightened risks for certain conditions including: IBS, acid reflux (GERD), bloating and gas, as well as constipation. Causes for increased gut issues at midlife include: slower digestion, microbiome imbalances, estrogen’s effect on water retention and gut motility. Strategies to reduce gut issues at midlife include: identifying trigger foods, mindful eating, and hydration.
Declining estrogen and progesterone levels can alter gut motility and visceral sensitivity, contributing to constipation and bloating. Studies indicate that postmenopausal women report higher rates of altered bowel function and IBS-like complaints compared to premenopausal women, with a peak in symptom prevalence during the perimenopausal years.
The gut microbiome composition also shifts during and after menopause. There is a trend toward reduced microbial diversity and a gut microbiome profile that becomes more similar to that of men, with specific taxa being depleted or enriched postmenopause. These changes may influence metabolic and inflammatory pathways, contributing to increased cardiometabolic risk. Gut permeability increases across the menopause transition, which is associated with heightened systemic inflammation and may contribute to comorbidities such as osteoporosis and metabolic syndrome. While hormonal changes are central, psychosocial factors such as stress, anxiety, and tension also significantly impact GI symptom severity during midlife. Racial and ethnic differences also influence the prevalence and severity of GI symptoms in midlife women. Overall, the interplay of hormonal decline, aging, changes in gut physiology, and psychosocial stressors underlies the increased GI symptom burden during the menopausal transition.
The Microbiome at Midlife
As women enter midlife, the gut microbiome often shows reduced diversity (the variety of species within a sample), making it less resilient to disruptions.This shift can begin as early as age 40, with the microbiome starting to resemble that of men more closely due to hormonal influences.Specific bacterial changes include increases in adverse organisms and and decreases in beneficial organisms. These compositional shifts are not uniform across all women and can vary by ethnicity, diet, and lifestyle, but they generally reflect a move toward a less protective microbial profile. Adverse shifts linked to estrogen deficiency include elevation of the Firmicutes/Bacteroidetes ratio and a drop in Bifidobacterium.
The function of the gut microbiome also evolves in midlife, often tied to the "estrobolome"—the subset of bacteria that metabolize estrogens. With falling estrogen, there's decreased microbial β-glucuronidase activity, which normally helps recirculate estrogens in the body, leading to lower systemic hormone levels. Some studies note increased SCFAs (like acetate, propionate, and butyrate) from bacteria such as Odoribacter, which can have anti-inflammatory effects but may also contribute to issues if imbalanced. Reduced saccharolytic potential (ability to break down carbohydrates) leads to lower acetate and higher gas production, especially with fibers like inulin. Increased hydrogen sulfide from bacteria like Bilophila, which can damage gut mucosa, increase permeability ("leaky gut"), and promote inflammation. Changes in sulfate transport and other pathways that correlate with progesterone metabolites, potentially affect hormone retention.
Hormone therapy (e.g., estrogen replacement) can partially reverse some of these, restoring diversity and reducing harmful bacteria like Proteobacteria.
Health Implications of Midlife Gut Changes
Midlife gut changes are linked to broader health risks, as the microbiome interacts with immunity, metabolism, and the gut-brain axis and can impact the heart, bones, brain, mental health and multiple other organs and their systems.
Altered gut microbiome composition correlates with higher blood pressure, lower HDL cholesterol, increased waist circumference, insulin resistance, and metabolic syndrome. For instance, enriched Sutterella is tied to elevated blood pressure, while depleted Clostridium lactatifermentans links to poorer lipid profiles. Dysbiosis contributes to osteoporosis and osteopenia via immune activation (e.g., increased TNF+ T cells and Th17 cells) and altered bone metabolism, with higher risks in women showing elevated Fusicatenibacter or Lachnoclostridium.
Increased inflammation may raise risks for non-alcoholic fatty liver disease, atherosclerosis, obesity, type 2 diabetes, depression, and even Alzheimer's. Reduced acetate could impair appetite regulation and immune responses.
Gut dysbiosis can indirectly affect vaginal and urinary microbiomes, exacerbating genitourinary syndrome of menopause (e.g., dryness, infections).
Despite these changes, a healthy gut microbiome is maintainable through diet (e.g., fiber-rich foods), probiotics, and lifestyle factors, potentially mitigating risks. Longitudinal studies emphasize that these shifts start subtly in midlife, underscoring the importance of early monitoring. If experiencing symptoms like bloating, irregular digestion, or related health issues, consult your physician and review your microbiome.
Gluten sensitivity does not have evidence to suggest that it specifically worsens in women during midlife. However, current literature highlights a gap in high-quality, prospective data directly addressing the impact of midlife or menopause on gluten sensitivity in women. Non-celiac gluten sensitivity (NCGS) is more frequently reported in adult women, particularly between ages 30 and 50, but this reflects a higher prevalence in this demographic rather than a documented worsening of symptoms during midlife or menopause. The clinical presentation of NCGS includes both gastrointestinal (e.g., abdominal pain, bloating, diarrhea, constipation) and extraintestinal symptoms (e.g., headache, fatigue, mood disturbances, brain fog), which can fluctuate and may be transient. There is no established data showing symptom exacerbation specifically related to midlife or hormonal changes. Notably, gynecological symptoms such as menstrual cycle alterations, recurrent vaginitis, and recurrent cystitis have been reported at higher rates in women with non-celiac gluten sensitivity compared to healthy controls and those with irritable bowel syndrome or celiac disease.
Current evidence suggests that small intestinal bacterial overgrowth does tend to get worse in women during midlife, with age and female gender both being independent risk factors, although the precise mechanisms and the impact of menopause or hormonal changes require further study. Multiple high-quality studies and guidelines indicate that both advancing age and female gender are associated with an increased risk of SIBO. Large cohort studies demonstrate that, in women, the odds of a positive SIBO breath test increase with age, whereas in men, the risk may decrease with age, suggesting a gender-specific effect of aging on SIBO risk. The increased risk in midlife women may be related to age-associated changes in gut motility, hormonal shifts, and a higher prevalence of comorbidities or medication use that can predispose to SIBO, although the exact mechanisms remain incompletely understood. Recent microbiome studies further confirm that the small intestinal microbiome changes significantly with age, with decreased diversity and increased abundance of potentially pathogenic bacteria. The most common symptoms of small intestinal bacterial overgrowth in women during midlife are bloating, diarrhea, and abdominal pain or discomfort. Extra-intestinal manifestations, including fatigue and poor concentration, have been described and may be more noticeable in midlife women, potentially reflecting subtle micronutrient deficiencies or systemic effects of chronic inflammation. However, it is important to note that no single symptom is pathognomonic for SIBO, and the clinical picture often overlaps with other functional gastrointestinal disorders, such as irritable bowel syndrome and functional dyspepsia.
Current evidence does not support a significant worsening of lactose intolerance in women specifically during midlife, as age-related increases in lactose malabsorption and intolerance symptoms are more pronounced in older age groups, and midlife women are not at clearly increased risk compared to other adults.
Common Gut Issues at Midlife
Irritable Bowel Syndrome (IBS)
Symptoms: Abdominal pain, diarrhea, constipation, or alternating patterns.
Triggers: Stress, diet, hormonal fluctuations.
Management: Low-FODMAP diet, stress reduction, and medical treatments.
Acid Reflux and GERD
Causes: Weakened lower esophageal sphincter, dietary triggers, stress.
Risks: Chronic GERD and esophageal damage.
Solutions: Dietary adjustments, meal timing, and lifestyle changes.
Constipation and Altered Gut Motility
Causes: Aging, dehydration, low fiber, sedentary lifestyle.
Hormonal factors: Progesterone’s effect on smooth muscle relaxation.
Solutions: Fiber-rich diets, exercise, and hydration.
SIBO (Small Intestinal Bacterial Overgrowth)
Causes:Slowed gut motility, low stomach acid, structural or anatomical issues, medications and lifestyle factors
Hormonal Factors:Reduced gut microbiome diversity, impaired motility
Solutions: Antibiotics, Low-FODMAP or low-carb diets starve bacteria, avoid triggers like sugar, herbal antimicrobials, probiotics/prebiotics for microbiome support; digestive enzymes, lifestyle and supportive measures.
Food Intolerances Including Lactose Intolerance
Causes: Stress and anxiety can predispose and worsen issues.
Hormonal factors : Fluctuating estrogen levels can increase histamine production, weaken gut lining integrity, and disrupt the gut-brain axis.
Solutions: Track your diet (e.g., elimination diets, blood tests), avoiding triggers, supplements like lactase enzymes or probiotics, and focusing on anti-inflammatory habits like exercise and stress reduction to potentially mitigate midlife changes.
Hormones and the Female Gut
Estrogen
Estrogen impacts the female gastrointestinal tract by modulating motility, visceral sensitivity, mucosal barrier integrity, immune responses, and epithelial secretion, with effects mediated through multiple estrogen receptor subtypes.
Estrogen generally inhibits GI motility, particularly in the colon, by activating on myenteric neurons, which increases nitric oxide release and suppresses smooth muscle contraction, leading to delayed colonic transit and reduced contractility. This effect is more pronounced in females and is modulated by hormonal fluctuations during the menstrual cycle. Estrogen also reduces chloride secretion in the colon, promoting fluid retention, especially during the luteal phase.
Estrogen influences visceral pain sensitivity and is implicated in the higher prevalence of disorders of gut-brain interaction (such as irritable bowel syndrome) in women. It enhances colonic hypersensitivity and modulates stress-related GI dysfunction, likely through both neuronal and immune pathways. Estrogen also affects immune cell recruitment and activation in the gut, contributing to sex- and age-dependent differences in inflammatory bowel disease pathogenesis.
Additionally, estrogen is associated with a protective effect against certain GI cancers, such as gastric and colorectal cancer. It also modulates the mucosal barrier and epithelial homeostasis, impacting susceptibility to inflammation and injury.
Fluctuating estrogen during perimenopause impacts the female gastrointestinal tract by increasing the prevalence and severity of gastrointestinal symptoms, altering gut motility, and disrupting the gut microbiome. During perimenopause, estrogen levels become erratic and ultimately decline, which is associated with increased reports of bloating, altered bowel habits (including constipation and diarrhea), and symptoms consistent with disorders of gut-brain interaction such as irritable bowel syndrome and functional dyspepsia.
These hormonal fluctuations can impair GI motility, leading to symptoms such as constipation and bloating, and may heighten visceral sensitivity, contributing to abdominal pain and discomfort. Additionally, perimenopausal estrogen changes are linked to increased gut permeability and low-grade inflammation, which may further exacerbate GI symptoms and contribute to systemic effects such as mood and cognitive disruption.
Estrogen fluctuations also disrupt the intestinal microbiome, promoting microecological imbalance and increasing susceptibility to GI and metabolic disorders. The overall effect is a higher burden of GI symptoms and reduced quality of life during the perimenopausal transition. The age groups most affected by gastrointestinal symptoms during perimenopause are women in their 40s to early 50s, which corresponds to the typical age range for the menopausal transition. Symptom prevalence, particularly for irritable bowel syndrome (IBS)-type complaints, peaks during the climacteric period, most notably between ages 40 and 49, and remains elevated into the early 50s as women transition through perimenopause and into early postmenopause.
During postmenopause, especially those in their mid-50s and beyond—continue to experience a high prevalence of gastrointestinal symptoms, but the pattern shifts toward more persistent altered bowel function, such as constipation, reflecting increased anorectal and pelvic floor dysfunction.
Menopausal hormone therapy (MHT) in perimenopausal and postmenopausal women has complex effects on gastrointestinal function. Estrogen replacement therapy (ERT) can modulate gut motility, visceral sensitivity, and the gut microbiome. In postmenopausal women, ERT has been associated with an increased risk of gastroesophageal reflux disease (GERD), likely due to estrogen-mediated relaxation of the lower esophageal sphincter and increased nitric oxide synthesis, which decreases smooth muscle tone and predisposes to reflux symptoms. This association is most pronounced with estrogen-only regimens. The most common side effects of estrogen replacement therapy on gastrointestinal health in perimenopausal and postmenopausal women are gastroesophageal reflux disease (GERD) symptoms (heartburn, regurgitation), nausea, abdominal pain, bloating, flatulence, constipation, and diarrhea. Multiple large cohort studies and meta-analyses demonstrate a significant association between estrogen therapy and increased risk of GERD, with estrogen-only regimens conferring the highest risk. This is thought to be due to estrogen-mediated relaxation of the lower esophageal sphincter, predisposing to reflux symptoms. While these side effects are generally mild to moderate, GERD symptoms may require intervention, and any new or severe gastrointestinal symptoms should prompt further evaluation to exclude other causes. The risk of these adverse effects should be considered when initiating estrogen replacement therapy in peri- and postmenopausal women.
Other frequently reported gastrointestinal adverse effects include nausea, abdominal pain, bloating, and flatulence, as well as constipation and diarrhea. Estrogen therapy is also associated with an increased risk of gallbladder disease and, rarely, cholestatic jaundice and pancreatitis.
ERT also influences the gut microbiome, partially restoring the diversity and composition that is lost after menopause, which may have beneficial metabolic and immunological effects, though the clinical significance of these changes remains under investigation. Current evidence does not support a consistent benefit of ERT for bowel symptoms such as constipation, diarrhea, or irritable bowel syndrome in peri- or postmenopausal women. Large cohort studies have not demonstrated a reduction in altered bowel function or IBS-type complaints with estrogen use.
Progesterone
Progesterone slows gastrointestinal (GI) motility, leading to delayed gastric emptying and prolonged intestinal transit time. This effect is well established in both non-pregnant and pregnant individuals and is a key contributor to symptoms such as constipation, bloating, and reflux, particularly during the luteal phase of the menstrual cycle and pregnancy. Increased progesterone during pregnancy reduces GI motility, lowers lower esophageal sphincter pressure (increasing risk for gastroesophageal reflux), and delays gastric emptying, which can result in constipation and related symptoms.
Mechanistically, progesterone acts directly on gut smooth muscle cells, in part by increasing nitric oxide synthesis and inhibiting contractile signaling pathways, resulting in smooth muscle relaxation and reduced peristalsis. Progesterone also modulates the function of interstitial cells of Cajal, further contributing to slowed colonic transit. Additionally, progesterone can reduce the contractile response of the gallbladder, predisposing to biliary stasis
Progesterone may also impact gut barrier function. It has been shown to upregulate tight junction proteins such as occludin, thereby decreasing intestinal permeability and potentially reducing systemic microbial translocation and inflammation during pregnancy. However, certain synthetic progestins may have adverse effects on epithelial integrity.
The most common gastrointestinal symptoms associated with progesterone use across different populations are constipation, abdominal pain or cramping, bloating (abdominal distension), nausea, diarrhea, and, less frequently, vomiting. These symptoms are consistently reported in both clinical trials of oral and vaginal progesterone formulations and in observational studies of women during periods of elevated endogenous progesterone, such as pregnancy and the luteal phase of the menstrual cycle.
In postmenopausal women and those receiving progesterone for hormone therapy or reproductive indications, clinical trial data show that abdominal pain, bloating, nausea, diarrhea, and constipation are the most frequently reported GI adverse effects, with rates for individual symptoms generally ranging from 2% to 20% depending on dose and formulation. In women with irritable bowel syndrome, GI symptoms such as constipation and abdominal pain may be exacerbated with progesterone use.
Testosterone
Testosterone impacts gastrointestinal health in women through several mechanisms, but its clinical significance remains incompletely defined. Endogenous testosterone appears to play a role in gastrointestinal motility and symptom modulation. Lower free testosterone levels in women have been associated with increased severity of irritable bowel syndrome (IBS) symptoms, and animal models demonstrate that androgen depletion impairs colonic motility, suggesting a physiological role for androgens in enteric neuronal regulation of bowel function.
Testosterone also influences the gut microbiota. Human and animal studies indicate that testosterone modulates the composition and diversity of the gut microbiome. In women, altered testosterone levels—such as those seen in polycystic ovary syndrome—are associated with distinct gut microbiota profiles.
DHEA
Dehydroepiandrosterone (DHEA) impacts the gut in women primarily through its immunomodulatory and anti-inflammatory effects, as well as by influencing gut microbiota composition and intestinal barrier function. Preclinical studies in animal models of colitis demonstrate that DHEA administration reduces intestinal inflammation by inhibiting pro-inflammatory cytokine production (such as TNF-α, IL-1β, and IL-6), blocking activation of NF-κB and p38 MAPK pathways, and suppressing NLRP3 inflammasome activation. DHEA also enhances intestinal barrier integrity by upregulating tight junction proteins and modulates the gut microbiota, notably decreasing the abundance of potentially pathogenic taxa such as Pseudomonas.
In women, DHEA is a precursor for sex steroids and is involved in local intracrine synthesis of androgens and estrogens in peripheral tissues, including the gut. While direct clinical data in women are limited, DHEA supplementation has been associated with improved immune function and reduced disease activity in inflammatory bowel disease, with pilot studies suggesting benefit in refractory Crohn’s disease and ulcerative colitis. However, the clinical efficacy and safety of DHEA supplementation for gut health in women remain to be fully established.
Additionally, in the context of polycystic ovary syndrome (PCOS), DHEA-induced androgen excess can contribute to gut dysbiosis, which may exacerbate metabolic and endocrine dysfunction, indicating that the impact of DHEA on the gut may be context-dependent. Overall, DHEA exerts pleiotropic effects on gut immunity, barrier function, and microbiota, but further research is needed to clarify its therapeutic role in women’s gastrointestinal health
Thyroid
The thyroid gland significantly impacts gastrointestinal health in women through both direct hormonal effects and immune-mediated mechanisms. Thyroid hormones regulate basal metabolic rate and gastrointestinal motility. Hypothyroidism commonly leads to reduced gut motility, resulting in symptoms such as constipation, delayed gastric emptying, and, in severe cases, ileus or pseudo-obstruction. Conversely, hyperthyroidism accelerates gastrointestinal transit, often causing diarrhea and increased bowel frequency. These effects are mediated by thyroid hormone action on intestinal smooth muscle and enteric nervous system function.
Autoimmune thyroid diseases, particularly Hashimoto's thyroiditis and Graves' disease, are frequently associated with other autoimmune gastrointestinal disorders, such as autoimmune atrophic gastritis and celiac disease. These autoimmune conditions can lead to malabsorption, micronutrient deficiencies (notably iron, vitamin B12, and others), and increased risk of anemia and malignancy. Women are disproportionately affected by these autoimmune conditions.
Alterations in the gut microbiota and increased intestinal permeability ("leaky gut") have been observed in women with autoimmune thyroid disease, suggesting a bidirectional relationship between thyroid function and gastrointestinal health. Dysbiosis may contribute to both thyroid autoimmunity and gastrointestinal symptoms, and dietary factors can modulate this axis
In summary, the thyroid gland influences gastrointestinal health in women via hormonal regulation of motility, immune-mediated associations with other GI diseases, and interactions with the gut microbiota, all of which can manifest as a spectrum of GI symptoms and nutritional deficiencies.
Cortisol
Cortisol has complex effects on gastrointestinal (GI) health in women, mediated through both physiological and stress-related mechanisms. Under normal conditions, cortisol contributes to the maintenance of gastric mucosal integrity and modulates immune responses in the GI tract. However, chronic or excessive cortisol exposure, as seen in stress or hypercortisolemic states, can disrupt GI homeostasis.
In women, dysregulation of the hypothalamic-pituitary-adrenal (HPA) axis and altered cortisol dynamics are implicated in the pathophysiology of functional GI disorders, particularly irritable bowel syndrome (IBS), which is more prevalent in females. Women with IBS often exhibit abnormal basal and stress-induced cortisol responses, including both blunted and exaggerated patterns, which are associated with altered visceral sensitivity, impaired intestinal barrier function, and changes in mucosal immune activity. These HPA axis alterations may contribute to symptom severity and comorbid anxiety, although the relationship is complex and not entirely linear.
Cortisol also impacts the gut microbiota. Chronic hypercortisolism, as seen in Cushing syndrome, is associated with gut microbial dysbiosis and the enrichment of cortisol-degrading bacteria, which may further influence host steroid metabolism and GI health. Additionally, stress-induced cortisol release can increase intestinal permeability, promote low-grade inflammation, and disrupt the gut-brain axis, exacerbating GI symptoms.
Sex hormones modulate the effects of cortisol on the GI tract, with evidence that estrogen and progesterone interact with glucocorticoid signaling, further influencing GI motility, sensitivity, and immune responses in women. This interplay may underlie the observed sex differences in GI disease prevalence and symptomatology.
FSH and LH
Follicle-stimulating hormone (FSH) and luteinizing hormone (LH) impact gut health in women primarily through their association with changes in the gut microbiome, particularly in the context of reproductive endocrine disorders. Elevated FSH and LH levels, as seen in conditions such as premature ovarian insufficiency (POI) and polycystic ovary syndrome (PCOS), are associated with significant alterations in gut microbial composition. In POI, increased FSH and LH correlate with reduced abundance of beneficial genera (e.g., Faecalibacterium) and increased abundance of potentially pathogenic genera (e.g., Sutterella, Dorea), suggesting that gonadotropin dysregulation is linked to gut dysbiosis and may contribute to systemic inflammation and metabolic dysfunction.
In PCOS, higher LH and LH:FSH ratios are positively correlated with increased levels of GABA-producing gut bacteria, which may influence neuroendocrine signaling along the gut-brain axis and further exacerbate reproductive and metabolic disturbances. Animal studies also indicate that interventions targeting the gut microbiota, such as administration of lactic acid bacteria, can modulate FSH and LH levels and improve ovarian and metabolic parameters, supporting a bidirectional relationship between gonadotropins and gut microbial health.
Overall, the current literature supports a model in which FSH and LH, through their effects on ovarian function and sex steroid production, indirectly modulate gut microbiota composition, while gut dysbiosis may in turn influence gonadotropin secretion and reproductive health.
Women’s Gut Health is Distinct From Men’s Gut Health
Gut health differs between women and men in several key aspects, primarily due to differences in sex hormones, gut microbiota composition, gastrointestinal (GI) motility, and disease prevalence.
Women generally have a higher prevalence of functional GI disorders such as irritable bowel syndrome (IBS) and functional dyspepsia, with more severe symptoms and greater comorbidity with anxiety and depression compared to men. Women are more likely to experience constipation and bloating, while men more often report diarrhea-predominant symptoms. These differences are partly attributed to slower gastric emptying and colonic transit in women, as well as increased visceral sensitivity, especially in premenopausal women, likely influenced by estrogen and progesterone fluctuations.
At the microbiome level, women typically exhibit a higher Firmicutes/Bacteroidetes (F/B) ratio, increased abundance of certain genera such as Akkermansia and Ruminococcus, and lower Bacteroidetes compared to men. These differences are most pronounced during reproductive years and tend to diminish after menopause, suggesting a strong hormonal influence. In men, higher levels of Prevotella and Fusobacterium are observed, and testosterone is associated with increased microbial diversity and specific taxa such as Ruminococcus and Acinetobacter. The gut microbiota also participates in the metabolism of sex hormones, creating a bidirectional relationship between hormones and microbial composition.
These sex-based differences in gut health may contribute to the observed disparities in disease susceptibility, symptom profiles, and responses to dietary or pharmacologic interventions between women and men.
The Impact of Hormone Support on Gut Health
Hormone therapy (HT), particularly in postmenopausal women, has several documented impacts on gastrointestinal health. HT is associated with an increased risk of gastroesophageal reflux disease (GERD), with both estrogen-only and combined estrogen-progestogen regimens showing higher odds of GERD symptoms and increased use of proton pump inhibitors compared to non-users. The mechanism is thought to involve hormone-induced relaxation of the lower esophageal sphincter, though the precise pathways remain incompletely defined. There is also evidence that MHT increases the risk of gallbladder disease.
Hormone therapy (MHT), particularly estrogen or combined estrogen-progestin therapy, confers several benefits relevant to gastrointestinal (GI) health in postmenopausal women. One notable benefit is a reduction in colorectal cancer risk associated with combined estrogen-progestin therapy.
In inflammatory bowel disease (IBD), MHT has been associated with improved disease activity in postmenopausal women, with studies showing a significant reduction in flare frequency and symptom severity, suggesting an anti-inflammatory role of estrogen in the GI tract. This protective effect may be mediated by estrogen’s modulation of immune responses and gut mucosal integrity.
MHT also influences the gut microbiome, which plays a critical role in GI health. Estrogen replacement can partially restore the gut microbial composition altered by menopause, potentially improving gut barrier function and reducing GI symptoms. However, the relationship between HT and gastroesophageal reflux disease (GERD) is complex; estrogen and progesterone may reduce lower esophageal sphincter tone, potentially increasing reflux risk.
The Gut Microbiome and Longevity
Multiple lines of evidence from human cohort studies, metagenomic analyses, and Mendelian randomization (MR) studies indicate that specific gut microbial taxa and their metabolic functions are associated with increased lifespan and healthy aging. Long-lived individuals, such as centenarians, consistently exhibit distinct gut microbiota signatures compared to younger adults, including enrichment of taxa such as Akkermansia, Alistipes, and certain species of Ruminococcaceae and Lachnospiraceae, as well as increased microbial diversity and functional capacity for short-chain fatty acid (SCFA) production and xenobiotic metabolism. MR analyses suggest potential causal relationships between the abundance of certain beneficial bacteria (e.g., Akkermansia muciniphila, Alistipes, Hungatella, Desulfovibrio) and longevity-related traits, while other taxa (e.g., Bacteroides massiliensis, Fusobacterium nucleatum) are negatively associated with lifespan.
Mechanistically, the gut microbiome may influence longevity through modulation of host metabolic, immune, and inflammatory pathways, including maintenance of gut barrier integrity, reduction of systemic inflammation, and production of metabolites such as SCFAs and indole derivatives that promote metabolic health and counteract age-related diseases. Animal studies further support a causal role, as fecal microbiota transplantation from healthy or long-lived donors can extend healthspan and lifespan in progeroid mouse models, with Akkermansia muciniphila identified as a key beneficial taxon.
In summary, current evidence supports a significant association—and likely a causal relationship—between gut microbiome composition and function and human longevity, with specific microbial taxa and metabolic pathways contributing to healthy aging and extended lifespan.
GI Cancers
Gastrointestinal (GI) cancers encompass a group of malignancies affecting the digestive system, including the esophagus, stomach (gastric), colon and rectum (colorectal), pancreas, liver, and biliary tract. Women face unique considerations, such as interactions with hereditary syndromes (e.g., Lynch syndrome, which also increases risks for endometrial cancer) and lifestyle factors like alcohol consumption limits. Colorectal cancer consistently ranks as the most common GI cancer in women. In the US, CRC is the third most common cancer in women (after breast and lung). Recent data show a gradual decline in overall incidence rates due to screening and lifestyle changes, but rates are rising among younger women (under 50), possibly linked to obesity, diet, and sedentary behavior. Incidence has risen by 2.4% annually in women under 50 and 0.4% in those 50-64 from 2012-2021. Globally, colon cancer incidence is higher in developed regions due to Western diets and aging populations.
The most common GI cancer in women is colorectal cancer (cancer of the large bowel). The lifetime risk of developing CRC is about 4.0% (or 1 in 25 to 1 in 26) for women, slightly lower than for men (4.3% or 1 in 23). CRC incidence increases with age, with the median diagnosis age at 66 years (both sexes combined, but similar for women). Early-onset GI cancers (diagnosed before age 50) are rising globally, particularly in women, driven by modifiable risks like obesity, poor diet, and sedentary lifestyles. Family history plays a critical role, as up to 10% of GI cancers are hereditary, linked to syndromes like Lynch (colorectal and endometrial), hereditary diffuse gastric cancer (HDGC with CDH1 mutations), and others such as FAP or Peutz-Jeghers. A detailed family history—including first- and second-degree relatives' cancer types, ages at diagnosis, and lineage—should be known for all women to assess risk, with red flags like multiple relatives with GI or related cancers (e.g., endometrial in Lynch) prompting genetic evaluation Understanding these cancers in women requires attention to gender-specific risks, such as lower alcohol thresholds for prevention and potential overlaps with reproductive health factors.
Early Warning Signs and Symptoms
GI cancers often present with subtle or nonspecific symptoms in early stages, making them challenging to detect. Symptoms can overlap with common digestive issues, but persistent or worsening signs warrant medical evaluation. In women, symptoms may sometimes be dismissed as related to gastrointestinal disorders like irritable bowel syndrome or hormonal changes, emphasizing the need for vigilance. A family history of GI cancers or hereditary syndromes should heighten awareness, prompting earlier investigation of even mild symptoms, as hereditary cases may present at younger ages.jamanetwork.comacog.org Below are key signs by major GI cancer types:
Esophageal Cancer: Early symptoms are rare, but may include difficulty swallowing (dysphagia), worsening indigestion or heartburn, chest pain, pressure, or burning, coughing or hoarseness, and unintentional weight loss. Advanced stages can cause more pronounced swallowing issues.
Stomach (Gastric) Cancer: Often asymptomatic early on, but signs can include trouble swallowing, abdominal pain, bloating after eating, feeling full after small meals, loss of appetite, heartburn, indigestion, nausea, vomiting (possibly with blood), unintentional weight loss, fatigue, and black stools. In women, these may mimic pregnancy-related or menopausal digestive changes. Additional symptoms like swelling or fluid buildup in the abdomen may occur if the cancer spreads.
Colorectal Cancer: Common early signs include changes in bowel habits (e.g., diarrhea, constipation, or narrower stools), rectal bleeding or blood in stool, abdominal cramps, gas, or pain, a sensation of incomplete bowel emptying, weakness, fatigue, and unexplained weight loss. In younger women, rectal bleeding and abdominal pain are key warning signs, appearing more frequently than in those without
Pancreatic Cancer: Typically silent in early stages, symptoms emerge later and include abdominal or back pain, loss of appetite, unintentional weight loss, jaundice (yellowing of skin/eyes), light-colored or floating stools, dark urine, itching, new or worsening diabetes, blood clots (causing arm/leg swelling), and fatigue. Women may experience these alongside endocrine-related issues if the cancer affects hormone production.
Liver Cancer: Early stages are often asymptomatic, but signs include unintentional weight loss, loss of appetite, upper abdominal pain, nausea, vomiting, weakness, fatigue, abdominal swelling, jaundice, white/chalky stools, fever, enlarged veins on the abdomen, and abnormal bruising/bleeding. In women, risks tie into conditions like non-alcoholic fatty liver disease, which is rising with obesity.
Early Warning Signs and Symptoms
General GI symptoms in women that shouldn't be ignored include ongoing abdominal pain, bloating, cramps, gas, indigestion, or pressure, which could indicate various GI cancers. For early-onset cases, symptoms may be vague, underscoring the importance of family history and genetic factors. For early-onset GI cancers, genetic testing is advised for all diagnosed under 50 to inform screening for family members.
Screening
Screening aims to detect precancerous changes or early cancers in asymptomatic individuals. Recommendations vary by cancer type, risk level, and guidelines. Women with family history, hereditary syndromes (e.g., Lynch or FAP), or conditions like Barrett's esophagus should start earlier. Family history assessment is essential, including details on relatives' cancers, ages at diagnosis, and polyps; red flags like multiple GI cancers in family prompt referral for genetic counseling. Genetic screening via multigene panels (testing genes like MLH1, MSH2, APC, CDH1, BRCA1/2) is recommended for high-risk women, especially those with early-onset diagnoses or syndromes like Lynch (which overlaps with gynecologic risks).
Esophageal Cancer: Screening is recommended for women with Barrett's esophagus (from chronic acid reflux) via endoscopy to examine the esophagus lining. No general population screening; discuss with a doctor if at high risk or family history of esophageal cancer.
Stomach Cancer: Not routinely recommended in low-risk areas like the U.S., but women with strong family history or hereditary risks (e.g., HDGC syndrome with CDH1) may undergo upper endoscopy and biopsy every 6-12 months starting 5-10 years before the family's earliest diagnosis. In high-incidence regions (e.g., East Asia), population screening via endoscopy starts at age 40-50. Genetic testing for CDH1/CTNNA1 is key for HDGC.
Colorectal Cancer: Start at age 45 for average-risk women (earlier if family history or syndromes like Lynch). Options include stool-based tests (e.g., multitarget stool DNA testing (mt-sDNA) every 3 years, demonstrating a 92% sensitivity for cancer), computed tomography colonography (CTC) every 5 years (89% sensitivity for larger polyps), colonoscopy every 10 years (reducing incidence by 46% and mortality by up to 95% over 12 years post-negative result), High-risk women (e.g., first-degree relative with CRC or Lynch syndrome) begin at age 20-25 or 10 years before the relative's diagnosis age, with more frequent intervals (every 1-2 years).
Pancreatic Cancer: No general screening, but high-risk women (strong family history or genetic mutations like BRCA1/2, PALB2) may have annual MRI or endoscopic ultrasound starting at age 50 or 10 years before the family's earliest case.
Liver Cancer: Women with cirrhosis, hepatitis B/C, or non-alcoholic fatty liver disease should have ultrasound and blood tests (e.g., AFP) every 6 months. Vaccination against hepatitis B is key for prevention in at-risk groups.cancer.gov.
Prevention
Prevention focuses on modifiable risk factors, as many GI cancers link to lifestyle, infections, and genetics. Women often have lower incidence than men but share risks like obesity and smoking; gender-specific guidelines include stricter alcohol limits (no more than 1 drink/day). Helicobacter pylori infection is a major factor for stomach cancer, treatable with antibiotics. Family history assessment guides prevention; women with hereditary risks should undergo genetic counseling for tailored strategies, such as prophylactic surgery (e.g., hysterectomy for Lynch, gastrectomy for HDGC).
Esophageal Cancer: Maintain healthy weight, eat fruits/vegetables, exercise 30+ minutes most days, limit alcohol (≤1 drink/day for women), quit smoking, and manage acid reflux to prevent Barrett's esophagus. Genetic counseling if family history suggests hereditary risks.
Stomach Cancer: Reduce salty/smoked foods, eat fruits/vegetables, quit smoking, treat H. pylori infections, and inform doctors of family history. In high-risk women (e.g., genetic mutations like CDH1), prophylactic gastrectomy may be considered after childbearing.
Colorectal Cancer: Diet rich in fruits, vegetables, whole grains; limit alcohol (≤1 drink/day for women); quit smoking; exercise regularly; maintain healthy weight. High-risk women may use aspirin or NSAIDs under medical supervision; prophylactic colectomy for syndromes like FAP.
Pancreatic Cancer: Quit smoking, maintain healthy weight via diet/exercise (focus on vegetables/fruits/whole grains). No proven dietary supplements, but controlling diabetes helps. For hereditary risks (e.g., BRCA), enhanced surveillance.
Liver Cancer: Limit alcohol (≤1 drink/day for women), maintain healthy weight, vaccinate against hepatitis B, prevent hepatitis C (safe sex, avoid shared needles), treat chronic hepatitis. Avoid obesity to reduce fatty liver risk.
Overall, for early-onset GI cancers in women; obesity, ultraprocessed foods, sugar-sweetened beverages, sedentary behavior, smoking, and alcohol should be addressed. Genetic counseling for syndromes like Lynch (15-30% of early-onset cases) aids prevention. Eradication of H. pylori and improved hygiene have driven global declines.
Diagnosis
Diagnosis involves a combination of history, physical exams, imaging, and biopsies. Women with symptoms should seek prompt evaluation, as delays can occur due to overlapping non-cancer causes. Family history is vital for diagnosis; include a three-generation pedigree to identify hereditary patterns.Genetic screening, including germline and somatic testing (e.g., multigene panels for Lynch genes, CDH1), is recommended for all early-onset cases or those with family history to guide treatment and family screening.
Colon Polyps
Colon polyps are growths that protrude from the mucous membrane lining the colon or rectum. They can vary in size, shape, and number, ranging from small, benign bumps to larger growths that may pose health risks. Polyps are typically detected during routine screenings like colonoscopies, as they often do not cause noticeable symptoms in their early stages. They can be classified into different types based on their histology and potential for malignancy, with some being more likely to progress to cancer if left untreated.
Colon polyps are relatively common, particularly in older adults. Studies estimate that about 15-40% of adults over the age of 50 in the United States have at least one colon polyp. The prevalence increases with age, and men are slightly more likely to develop polyps than women. Risk factors such as a family history of polyps or colorectal cancer, obesity, smoking, and a diet high in red or processed meats can increase the likelihood of developing polyps. Regular screening is recommended, especially for those over 45 or with risk factors, to detect and remove polyps early.
Most colon polyps are asymptomatic, meaning they do not cause noticeable symptoms, which is why screening is critical for early detection. However, larger polyps or those that cause irritation may lead to symptoms such as rectal bleeding, blood in the stool (which may appear bright red or dark), changes in bowel habits (diarrhea or constipation lasting more than a few weeks), abdominal pain, or a feeling of incomplete bowel evacuation. In rare cases, large polyps can cause obstructions or significant bleeding, requiring urgent medical attention.
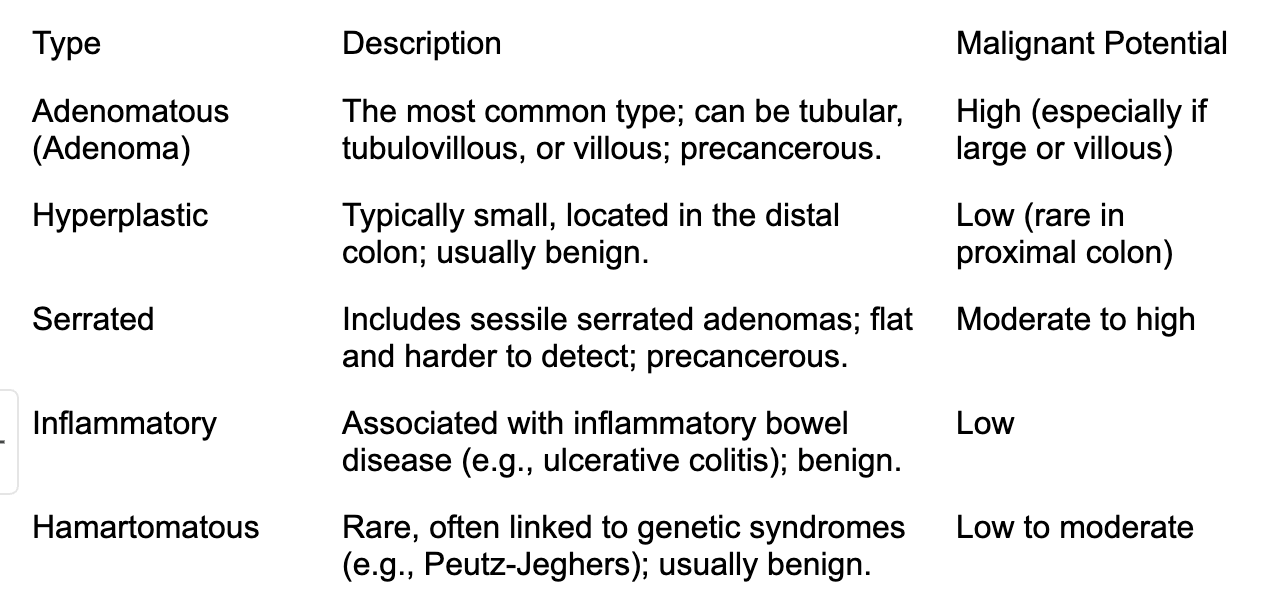
Not all colon polyps progress to cancer, but certain types have a higher risk. Adenomatous polyps (adenomas) are the most likely to become cancerous, with the risk depending on their size, number, and histological features. Larger adenomas (over 1 cm) or those with villous features are more concerning. Serrated polyps, particularly sessile serrated adenomas, also carry a cancer risk. Hyperplastic polyps are generally benign but can occasionally progress if located in the proximal colon. The progression to colorectal cancer typically occurs over several years, which underscores the importance of regular screening and polyp removal to prevent malignancy.
The following table summarizes the main types of colon polyps, their characteristics, and their potential for malignancy.
The Impact of Medication on Gut Health
Nonsteroidal anti-inflammatory drugs (NSAIDs) like ibuprofen and aspirin can irritate the stomach lining, potentially causing ulcers or gastritis, and disrupt the gut microbiome, increasing inflammation. Antibiotics, while targeting infections, often kill beneficial gut bacteria, leading to dysbiosis, diarrhea, or Clostridium difficile infections. Proton pump inhibitors (PPIs), used for acid reflux, reduce stomach acid, which may impair digestion and increase the risk of small intestinal bacterial overgrowth (SIBO) or infections. Opioids slow gut motility, causing constipation or narcotic bowel syndrome. Metformin, commonly prescribed for diabetes, can cause diarrhea or bloating by altering gut microbiota or increasing intestinal glucose exposure. Chemotherapy drugs and immunosuppressants may damage the gut lining, leading to mucositis or increased infection risk. Corticosteroids, like prednisone, can disrupt microbial balance and increase susceptibility to GI infections. Even over-the-counter laxatives, if overused, can disrupt natural bowel function. Patients experiencing persistent GI symptoms should consult a healthcare provider to assess medication effects, consider alternatives, or incorporate gut-supportive strategies like probiotics.
Assess Your Gut Health: Tests to Do
Know Your Family History and Have Genetic Testing if Indicated
Family history is a critical risk factor for gastrointestinal (GI) cancers, such as colorectal, gastric, and pancreatic cancer, as certain genetic mutations, like those in Lynch syndrome or BRCA genes, significantly increase susceptibility. Individuals with a first-degree relative (parent, sibling, or child) diagnosed with a GI cancer, especially at a young age (under 50), or those with multiple relatives affected across generations, should consider genetic testing. Additional red flags include a family history of related cancers (e.g., breast, ovarian) or known hereditary syndromes. Genetic testing, typically guided by a genetic counselor, involves analyzing DNA for mutations linked to hereditary cancer syndromes and is recommended for those with strong family histories to assess risk and inform screening or preventive strategies.
Polygenic risk scores (PRS) are emerging tools that estimate an individual’s genetic predisposition to gastrointestinal (GI) cancers, such as colorectal, gastric, or pancreatic cancer, by analyzing multiple genetic variants across the genome. Unlike single-gene mutations linked to hereditary syndromes like Lynch syndrome, PRS aggregate the effects of many low-risk genetic markers to provide a cumulative risk assessment. For GI cancers, PRS can help identify individuals at higher risk, even without a strong family history, enabling personalized screening strategies, such as earlier or more frequent colonoscopies. However, PRS are not yet widely used in clinical practice for GI cancers due to limitations in predictive accuracy across diverse populations and the need for further validation, but they hold promise for refining risk stratification and prevention efforts.
Get Screened
Screening for colon cancer is a vital preventive measure that significantly reduces mortality by detecting precancerous polyps or early-stage cancer when treatment is most effective. Recommended screening methods include colonoscopy, which allows visualization and removal of polyps every 10 years starting at age 45 for average-risk individuals, as well as less invasive options like stool DNA tests every 3 years. For those with a family history of colon cancer or hereditary syndromes like Lynch syndrome, screening may begin earlier (e.g., age 40 or younger) and occur more frequently, guided by a healthcare provider. Regular screening, tailored to individual risk factors, is crucial for early detection and improved outcomes.
Gut Microbiome Testing
Gut microbiome tests are widely available for microbiome assessment for direct purchase. These tests may have utility to assess your microbiome; however, additional study, standardization, regulatory oversight, and further research are needed before these tests can be considered reliable for routine clinical practice. These tests provide insights into microbial diversity and balance, which may relate to digestion, immunity, and overall health. However, they're not diagnostic tools and should not replace professional medical advice.
Key factors to look for:
Type of Analysis and Sequencing Method: Opt for kits using advanced techniques for more accurate, comprehensive results. Avoid basic tests if you want insights beyond just bacteria, as they may miss fungi, viruses, parasites, or archaea.
What the Test Covers: Ensure it aligns with your goals, such as gut health (most common), oral, vaginal, or full-body insights. Look for tests that screen for a wide range (e.g., over 120,000 microbes including bacteria, fungi, viruses, and parasites in Tiny Health) and provide scores for "good" vs. "bad" bacteria, diversity, or specific markers like zonulin for leaky gut.
Actionable Insights and Results: The best kits offer easy-to-understand reports with personalized recommendations, like diet plans, food suggestions, or custom probiotics/supplements.
Accuracy and Scientific Backing: Choose kits from reputable companies with FDA registration, GMP certification, or peer-reviewed methods.
Privacy and Data Security: Review policies on data storage and sharing. Look for kits that anonymize data and allow opt-outs for research.
Customer Support and Reviews: Seek kits with responsive support, consultations.
Limitations to keep in mind:
These tests can't diagnose conditions, detect if microbes are alive/dead, or replace clinical care. Results fluctuate and may not be useful for doctors yet due to limited tech integration.
Start by defining your health goals, then compare a few kits.
SIBO testing
At-home small intestinal bacterial overgrowth (SIBO) kits are not considered fully reliable due to several limitations inherent to the home testing environment, but can be useful. These kits, which typically use glucose or lactulose substrates for breath testing, offer convenience and noninvasiveness. Breath testing in general—including both at-home and in-clinic kits—has variable sensitivity and specificity, and further validation of at-home testing methods is needed. The glucose breath test is preferred over lactulose due to a lower rate of false positives.
Evidence-Based Interventions for Optimizing GI Health
Nutrition Strategies
High-fiber, plant-rich diets (including prebiotics and Mediterranean dietary patterns) are consistently supported for optimizing GI health. For fiber intake aim for 25–30 g/day. High-prebiotic diets have demonstrated significant benefits for both GI and psychological health, likely mediated by enrichment of beneficial gut bacteria and increased SCFA production. The Mediterranean diet is associated with increased microbial diversity and anti-inflammatory effects.
• Low-FODMAP Diet: The low-FODMAP diet, which restricts fermentable oligosaccharides, disaccharides, monosaccharides, and polyols, is strongly recommended for those with gut symptoms that have not been associated with more significant issues. There is robust evidence for reducing GI symptom burden and may also positively influence mood and gut microbiota composition. FODMAPs are certain types of carbohydrates found in foods like certain fruits, vegetables, grains, and dairy products. When eaten in large amounts, these carbs can cause discomfort for some individuals. By reducing or avoiding high FODMAP foods for a period, many people experience relief from their symptoms. After this initial phase, foods are gradually reintroduced to identify which ones trigger symptoms, helping to create a personalized eating plan that supports better digestion and overall comfort. On a low FODMAP diet, you'll need to avoid or limit foods that are high in certain fermentable carbs, like some fruits, vegetables, grains, dairy, and sweeteners. For example, avoid apples, pears, watermelon, garlic, onions, wheat, rye, and certain dairy products like milk and soft cheeses that contain lactose. Instead, focus on low FODMAP options such as bananas, strawberries, carrots, spinach, rice, oats, lactose-free dairy, and gluten-free bread. It's helpful to work with a healthcare professional or dietitian to create a balanced plan and ensure you're getting all the necessary nutrients while following the diet.
• Fermented Foods: Fermented foods (yogurt, kefir, kimchi, sauerkraut) increase gut microbial diversity and decrease inflammatory markers in healthy adults.
• Prebiotics and Probiotics: Prebiotics (non-digestible food ingredients that stimulate beneficial gut bacteria) and probiotics (live microorganisms that confer health benefits) are supported by clinical trials for their role in enriching beneficial bacteria, increasing SCFA production, and improving markers of GI, metabolic, and cognitive health. Thought for 70s
For women in midlife, typically encompassing perimenopause and menopause (around ages 40-60), probiotics can support health by addressing changes in the gut and vaginal microbiomes driven by declining estrogen levels. These shifts may contribute to symptoms like hot flashes, night sweats, mood changes, vaginal dryness, digestive issues, reduced bone density, and altered immune function. A recent July 2025 meta-analysis of 39 studies involving over 3,100 women found that probiotics have promising effects on alleviating total menopausal symptoms, vasomotor issues (e.g., hot flashes), psychological symptoms, vaginal dryness, and microbiome health, though results were mixed for somatic or sexual symptoms, and more high-quality research is needed due to study biases.pubmed.ncbi.nlm.nih.gov Probiotics don't replace hormone therapy but can complement lifestyle changes like a fiber-rich diet and exercise.
Probiotic supplementation, particularly with Lactobacillus and Bifidobacterium strains, may improve psychological or biological measures of depression, anxiety, or stress, especially in individuals predisposed to mood disorders.
When looking for a probiotic choose:
Strain Specificity
Look for the full identification of the probiotic strains, including genus, species, and strain (e.g., Lactobacillus rhamnosus GG or Bifidobacterium longum BB536). Different strains offer different benefits—some help with IBS, others with antibiotic-associated diarrhea or vaginal health.
Choose strains backed by clinical research for your specific condition or goal. For instance, if you're targeting gut issues, opt for strains like Lactobacillus acidophilus or Bifidobacterium bifidum that have shown efficacy in human trials.
Colony-Forming Units (CFUs)
Check the CFU count, which indicates the number of live organisms per dose. Aim for at least 1–10 billion CFUs for general use, but higher (up to 50 billion or more) may be needed for certain conditions—though more isn't always better, as it depends on the strain's potency.
Ensure the label specifies the CFU amount at the time of expiration, not just manufacturing, to guarantee viability.health.
Quality and Third-Party Testing
Select supplements from reputable brands with third-party verification (e.g., USP, NSF, or ConsumerLab seals) to confirm purity, potency, and absence of contaminants like heavy metals or allergens.
Check for unnecessary additives, fillers, or sugars that could counteract benefits. If you have dietary restrictions, ensure it's vegan, gluten-free, or non-GMO as needed.
Form and Delivery Method
Consider the format: Capsules or tablets are common and convenient, but powders or liquids might suit better for mixing into food. For targeted delivery (e.g., to the colon), choose time-release options.
If you're new to probiotics, start with a multi-strain product for broad benefits, but consult a healthcare provider for personalized advice.cdhf.cawebmd.com
Potential Side Effects and Precautions
Most people tolerate probiotics well, but watch for mild side effects like gas or bloating initially. Those with weakened immune systems or serious illnesses should consult a doctor before starting.
Probiotics aren't regulated as strictly as drugs by the FDA, so rely on evidence from randomized controlled trials rather than hype.
Probiotics from food sources like yogurt, kefir, sauerkraut, or kimchi can be a natural alternative or complement to supplements. Always talk to a healthcare professional, especially if you have underlying health issues, to ensure the choice aligns with your needs.healthline.comwebmd.com
Lifestyle Interventions: Regular physical activity, stress management, and adequate sleep and exercise are integral to GI health and its downstream effects on energy, mood, and immunity. Adequate hydration, limiting caffeine and alcohol, and creating time for relaxation and leisure are all important to great gut health.
Stress management: When you're stressed, your body produces more cortisol, which can interfere with normal digestion by slowing down gut movements, increasing inflammation, and making the gut lining more sensitive. This can lead to symptoms like stomach pain, bloating, and changes in bowel habits such as diarrhea or constipation. Chronic high levels of cortisol can also weaken the gut’s protective barrier, making it easier for harmful substances to enter the bloodstream and potentially contributing to digestive disorders. Managing stress and keeping cortisol levels balanced can help support a healthier gut and improve digestion.
Stress reduction techniques: Mindfulness, yoga, and breathing exercises.
Sleep: When you sleep well, your body has a chance to repair and regulate your digestive system, helping to keep things moving smoothly. Poor or insufficient sleep can disrupt the balance of good bacteria in your gut, slow down digestion, and increase inflammation, which may lead to issues like bloating, stomach pain, or irregular bowel movements. Additionally, lack of sleep can affect hormones that control hunger and appetite, making you more likely to overeat or crave unhealthy foods, which can further upset your gut health. So, making sure you get enough restful sleep is a key step in maintaining a happy, healthy gut.
Sleep hygiene tips to support the gut-brain axis.
Exercise: Regular exercise influences gut health through several mechanisms. Physical activity stimulates the muscles of the gastrointestinal tract, enhancing motility and helping food move smoothly through the digestive system. It also increases blood flow to the gut, promoting better nutrient absorption and tissue health. Moreover, exercise can modulate the immune system and reduce inflammation, which benefits gut lining integrity. Additionally, physical activity has been shown to promote a diverse and balanced gut microbiome, supporting beneficial bacteria that aid digestion and protect against harmful pathogens. These combined effects help maintain a healthy, functional gut environment.
Exercise routines for gut health: Walking, strength training, and yoga.
Supplements and Natural Remedies
Prebiotics are non-digestible fibers or compounds found in certain foods that serve as nourishment for beneficial bacteria already present in your gut. When you consume prebiotics, they pass through your digestive system largely intact and reach your colon, where they are fermented by gut bacteria. This process helps increase the number and activity of good bacteria, supporting overall gut health and digestion. Common sources of prebiotics include foods like garlic, onions, leeks, asparagus, bananas, and chicory root.
Probiotics are beneficial live bacteria that, when ingested in adequate amounts, can help improve or restore the natural balance of bacteria in your gut. These good bacteria can aid digestion, boost immune function, and potentially reduce symptoms of gastrointestinal disorders. You can find probiotics in fermented foods such as yogurt, kefir, sauerkraut, kimchi, and certain dietary supplements. Different strains of bacteria, like Lactobacillus and Bifidobacterium, are commonly used as probiotics.
Synbiotics are a combination of probiotics (live beneficial microorganisms, like bacteria or yeast) and prebiotics (non-digestible fibers that feed those microorganisms), designed to work together to promote gut health. Probiotics help colonize the gut with beneficial microbes, while prebiotics provide the nourishment to support their growth and activity. This synergistic effect can improve digestion, boost immunity, and support overall health. Examples include yogurt with added inulin or supplements combining Lactobacillus strains with fructooligosaccharides (FOS).
Postbiotics are the byproducts or bioactive compounds produced when beneficial bacteria ferment prebiotics or digest nutrients.Postbiotics may include inactivated microbial cells, cell components (such as cell wall fragments, peptidoglycans, teichoic acids), and microbial metabolites (such as short-chain fatty acids, enzymes, and peptides) in a non-purified, inactivated cell preparation. These components can be generated through various inactivation methods, including heat, radiation, or chemical processes.[ These substances, such as short-chain fatty acids (like butyrate), lactate, and other bacterial metabolites, can exert positive effects on gut health even without the presence of live bacteria. Postbiotics can help reduce inflammation, strengthen the gut lining, and support immune responses, making them an emerging area of interest for promoting gut health and overall well-being. Importantly, because postbiotics do not contain live microorganisms, they offer a favorable safety profile, particularly in populations where probiotic use may be contraindicated
Sodium butyrate, a form of butyrate (a short-chain fatty acid produced by gut bacteria), shows promise in helping with bloating, particularly when it's related to conditions like irritable bowel syndrome (IBS) or gut inflammation. Research indicates it can reduce symptoms such as abdominal discomfort, flatulence (gas and bloating), and irregular bowel movements by supporting the gut barrier, modulating the microbiome, reducing inflammation, and improving intestinal motility and receptor sensitivity.
Dietary fiber (e.g., psyllium) is effective for improving bowel regularity and symptoms in functional GI disorders such as IBS and chronic constipation. Dietary fiber (soluble and insoluble) is well established to improve bowel regularity, support microbial diversity, and reduce symptoms in functional GI disorders.
Micronutrients such as vitamin D and selenium have been shown to modulate the gut microbiome and support gut barrier function, with vitamin D in particular enhancing anti-inflammatory effects and microbial diversity. Omega-3 fatty acids and polyphenols have anti-inflammatory and microbiome-modulating effects.
Digestive enzyme supplements are oral preparations containing exogenous enzymes such as amylase, protease, lipase, and lactase, designed to aid the breakdown of macronutrients in the gastrointestinal tract. These supplements are primarily indicated for specific enzyme deficiencies, such as pancreatic exocrine insufficiency or lactase deficiency, where they have established efficacy. There is emerging but preliminary evidence from animal and short-term human studies suggesting that exogenous enzymes may modulate gut microbiota and improve digestive symptoms in individuals with mild complaints.
Peppermint oil has the strongest evidence among the three agents for gastrointestinal symptom relief, particularly in irritable bowel syndrome (IBS) and functional dyspepsia. Peppermint oil acts via smooth muscle relaxation and modulation of visceral sensation. Adverse events are generally mild (e.g., heartburn), but occur more frequently than with placebo. Typical dosing in clinical trials is enteric-coated capsules containing 180–225 mg, taken two to three times daily, though regimens vary and should be individualized.
Ginger is well-supported for antiemetic effects, especially in pregnancy, postoperative, and chemotherapy-induced nausea and vomiting, with high-quality evidence for these indications. There is some evidence for benefit in dyspepsia and indigestion, but data for broader gastrointestinal symptom relief in otherwise healthy adults are limited and mixed. Effective doses in studies range from 0.5–3 g/day in capsule form, administered for up to 3 months.
Chamomile has a long history of traditional use for gastrointestinal complaints.
Know Risk Factors and Early Warning Signs of Impaired GI Health
The most common risk factors include:
Poor diet (low fiber, high processed foods), physical inactivity, high BMI, psychological stress, female sex, prior gastroenteritis, environmental exposures (toxins, food additives), smoking, alcohol use, chronic inflammatory conditions, and certain medications.
Early warning signs:
GI symptoms (abdominal pain, bloating, altered bowel habits)
Fatigue
Mood disturbances
Immune-related manifestations (increased infections, low-grade inflammation)
Nutritional deficiencies
Overlap of multiple symptoms
Prevention and management:
Prevention and early management of GI dysfunction in adults are critical determinants of long-term outcomes in energy, mood, and immune-related health. Timely, evidence-based interventions that address the multifactorial nature of GI dysfunction—encompassing dietary, lifestyle, microbiota, and psychological domains—are associated with improved physical functioning, enhanced psychological well-being, and greater immune resilience.
Early intervention reduces the risk of chronic, fluctuating symptoms that are otherwise associated with impaired quality of life, increased healthcare utilization, and diminished work productivity.
Red flags that require evaluation:
Unexplained weight loss
Blood in stool
Persistent pain
Summary
Building sustainable habits for a personalized GI health plan involves setting realistic goals for diet, exercise, and stress management while addressing barriers like time constraints and social pressures. A tailored GI health plan might include incorporating fiber-rich foods, such as fruits, vegetables, and whole grains, to support digestion, alongside regular physical activity, like 30 minutes of moderate exercise most days, to promote gut motility. Stress management techniques, such as mindfulness or yoga, can reduce GI symptoms exacerbated by anxiety. Overcoming barriers requires planning, like meal prepping to save time or communicating health goals to friends to navigate social settings. Consistency, small incremental changes, and professional guidance from a dietitian or healthcare provider ensure long-term success in maintaining GI health.
A healthy gut is pivotal for enhancing quality of life and reducing the risk of chronic diseases such as colorectal cancer, diabetes, and cardiovascular disease, as a balanced gut microbiome supports immunity, metabolism, and inflammation regulation. As women transition through perimenopause and beyond, hormonal changes can impact gut motility and microbial diversity, necessitating tailored strategies to maintain GI health. Incorporating a diet rich in fiber from fruits, vegetables, and whole grains promotes regular bowel movements and feeds beneficial gut bacteria, while fermented foods like yogurt or kefir can enhance microbiome diversity. Regular exercise, such as walking or yoga, supports gut motility and reduces stress, which is crucial since menopause can heighten stress-related GI issues like irritable bowel syndrome. Staying hydrated and limiting processed foods or excessive alcohol mitigates inflammation and supports digestion. For women, bone health is critical, so calcium-rich foods paired with vitamin D from safe sun exposure or supplements aid both gut and skeletal health. Addressing individual needs, such as managing bloating or food sensitivities, ensures a sustainable plan. Overcoming barriers like time constraints or social dining pressures involves meal planning and choosing gut-friendly options in social settings. Probiotics or prebiotics may be considered to restore microbial balance, especially if antibiotics or medications disrupt the gut. By prioritizing these habits, women can reduce the long-term risk of GI-related chronic conditions, improve energy levels, and enhance mental clarity. Consistent small steps, like daily fiber intake or stress-reducing practices, compound over time, enabling women to age with confidence and a thriving gut ecosystem.
5 Habits for Great Gut Health
Increase Fiber Intake: Aim for 25–30 g/day from sources like lentils, oats, leafy greens, and berries to support gut motility and microbial diversity.
Incorporate Probiotics and Prebiotics: Eat fermented foods (yogurt, kefir, sauerkraut) and prebiotic-rich foods (whole grains, flaxseeds) to nourish beneficial gut bacteria.
Manage Stress: Practice mindfulness or yoga, as stress can exacerbate digestive issues like IBS, which is prevalent in midlife women.
Stay Hydrated: Drink 4–6 cups of water daily to prevent constipation, a common issue in midlife due to slower gut motility.
Consider Probiotic Supplements: Look for strains like Lactobacillus rhamnosus GG or Bifidobacterium longum BB536 with 1–10 billion CFUs for general gut health.